Technology
ABOUT THE TECHNOLOGY
The treatment involves packing industrial components and specially formulated metal powders into a sealed container or a retort. The retort is placed in a furnace and heated to a specific temperature for a specified length of time. Chemical reactions create a transport mechanism by which aluminum is introduced into the surface of the component. This produces an inter-metallic alloy layer that provides protection against high-temperature corrosion, and erosive wear. This pack cementation process is usually called calorizing, aluminizing, or alonizing.
There are many benefits of this technology. The introduction of aluminum into the surface of a base metal, seals the base metal. This process does not allow oxygen and other gases to diffuse into the micro-structure and cause corrosion.
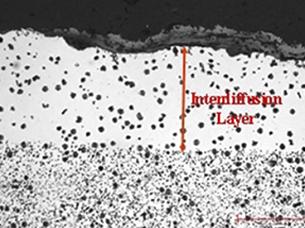
Diffusion processing results in the formation of the desired alloy on the surface where it is the most needed. Through diffusion it is possible to get higher concentrations of the desired element in the resulting alloy than through direct alloying. This can increase the material’s resistance to corrosion.
Diffusion is not a line-of-sight technology. The protective layer can be created on the inside of pipes and on components with a complex shape that are difficult to protect using a coating process.
The alloy layer formed is not a coating. Due to the establishment of a metallurgical bond, the alloy layer cannot be chipped off. The protective layer can only be removed through machining.
Surface Hardness
The hardness of the diffused layer is significantly harder(normally 4-5 times) than the original substrate metal.

Oxidation Resistance
Excellent oxidation resistance and no scaling.
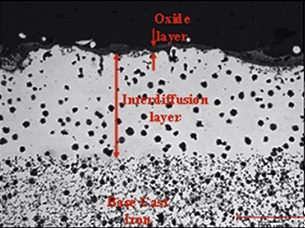
The protection mechanism is that under oxidizing conditions the diffused aluminum alloy reacts with oxygen to form aluminum oxide (Al203). Aluminum oxide is an extremely stable material and acts as a surface ceramic barrier. Accordingly, the base material is protected against oxidation.
1200ºC for 10 hours
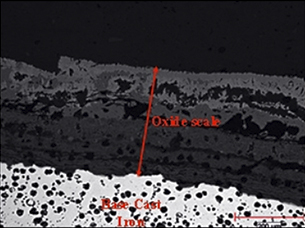
Reduction in surface oxidation - High silicon nodular cast iron
1010ºc-1020ºc gas temp
930ºC-940ºC peak metal temp
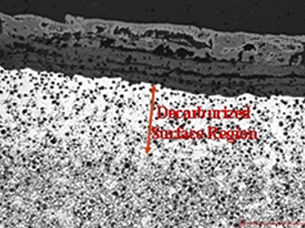
Reduction in decarburization - High silicon nodular cast iron
1010ºc-1020ºc gas temp
930ºC-940ºC peak metal temp
Carburation Resistance
Calorized parts have a unique resistance to carburation. When exposed to high temperatures at carbon-rich atmospheres, steels will be carburized, and therefore become brittle and less corrosion resistant. The protection mechanism is that iron-aluminum alloy formed on the surface has a lower solubility for carbon as compared to an unprotected surface.
Hydrogen Permeation Resistance
Energy producing companies have conducted research on hydrogen permeation of different tubing materials and found that calorizing reduced hydrogen permeation by a factor of 1000.
Sulphidation Resistance
Calorized surfaces exhibit complete resistance to corrosion caused by exposure to H2S, SO2 and SO3 at temperatures in excess of 400F. Under these sulphidizingconditions, calorized components will not scale.
Low Impact Abrasion/Erosion Resistance
Because the surface hardness of a calorized part is significantly higher than the base material, the resistance to abrasion is improved. Due to the metallurgical bond between the calorized layer and the base metal, the calorized layer will not spall nor peel off.
Preventing Thermal Fatigue
For applications such as water-cooled furnace components in which one surface is exposed to extremely high temperatures and the other is cooled by water or air, repeated expansion and contraction of the surface occurs.
Then the water-cooled component due to the changes in the operation temperature is subject to cyclic tensile and compressive stresses.
Often, due to temperature gradient between the inner and outer layers, cracking occurs on the surface. The cracked surface is then subjected to oxidation and other forms of corrosion that permits access of hot gases to deeper layers of the component. The cracking gradually progresses into the mass until the component fails.
Calorizing imparts resistance to oxidation and sulphidation from furnace atmospheres, due to the aluminum-rich surface layers created by the calorizing process. The aluminum oxidizes preferentially to extremely stable alumina that retards the formation of other oxides on the sidewall of open cracks, thus reducing the rate of propagation of the cracks. Additionally, both the alumina and the aluminum-rich alloy surface layers of the calorized components exhibit low thermal conductivity.
Charactersitic of calorizing
In-Soluble characteristic of calor
izing with molten metals
Our aluminum oxide layer created by calorizing is a very hard and extremely stable refractory material.
A North American steel research institute tested our calorized copper plates by pouring molten iron on top of it. The normal copper plate burned through within a second. The titanium or inconel welded copper burned through within 5 to 7 seconds. However, our calorized copper never burned through during the testing period.